NAPA VALLEY, Calif. — Wine has often been called one of humanity’s most valued art forms. It is the transformation of simple fruit juice into a sublime drink, a subject of poetry and romance. Even the Bible mentions it as a reward for a spiritual life.
Yet at a more basic level wine is essentially a complex chemical mixture. Before sealing the bottle, winemakers, many of whom are well-versed in chemistry, meticulously analyze it. They examine acidity, potassium levels, brettanomyces presence and other technical aspects.
Many details in this micro-analysis are crucial for producers but not particularly relevant for most consumers. For instance, if you favor a specific chardonnay, you’re likely not interested in its leucoanthocyanin levels. However, one significant statistic that is often overlooked but could aid in understanding wine better is its pH level.
Despite its importance, wineries seldom disclose the pH levels to consumers, fearing they might detract from the wine’s romantic allure. I’m also hesitant to write about such a technical subject, but the importance of pH in wine cannot be overstated. It is essential for balance; without the right pH, wines can quickly become unstable and deteriorate.
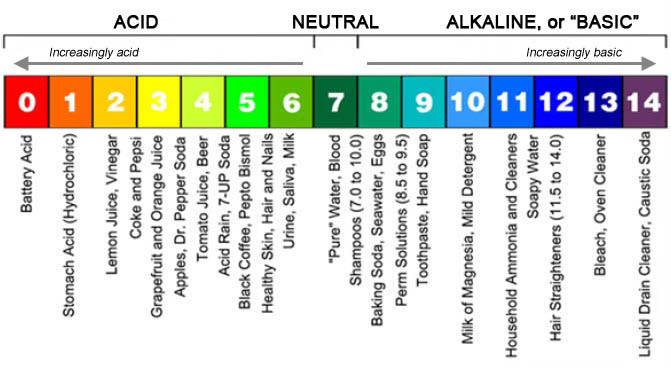
pH is a measure of how acidic or basic a solution is, with a range of 0 to 14. A pH of 7 is neutral, lower numbers indicate acidity and higher numbers indicate alkalinity. pH is an important concept in chemistry and many other fields, as it affects the behavior and properties of aqueous solutions. The term "pH" stands for "potential (or power) of hydrogen" and is a measure of the concentration of hydrogen ions in a solution.
As grapes grow, they start with high acidity and low pH, which gradually shifts as they ripen and sugars increase. Wines such as German riesling, French chablis and certain dessert wines that are known for their low pH are especially tart and can age well.
Few wineries reveal their wines’ chemical compositions, a trend that has led to a lack of technical understanding among consumers. I believe, however, that revealing pH levels could empower consumers to make more informed choices, especially when paired with food. For example, white wines with a pH around 3.5 or 3.6 might lack the necessary acidity, whereas those with a pH of about 3.2 or 3.3 are likely to be more balanced and ageable.
Alcohol content also plays a role, with lower-alcohol wines generally pairing better with food. In red wines, a pH of 3.5 to 3.7 is ideal for aging and balance. Historically, Napa Valley cabernets with pH in the 3.3 to 3.5 range were considered ideal and have aged beautifully.
However, high-pH wines tend to deteriorate quickly and may not develop mature characteristics. Wines with a pH above 3.85 are best consumed young. Balance is key in wine, and moderate alcohol levels (between 12% and 13.5%) have been successfully aged in the past.
Total acidity is another important factor, with a range of 5.5 to 7.0 grams per liter (g/L) being ideal for most wines in my experience. For young red wines to harmonize well with food, their acidity should be closer to 6.0 g/L, with a pH around 3.7.
Understanding a wine’s pH is just the beginning. It’s a crucial tool but not the only one for determining a wine’s potential excellence. A good example is the 2020 Freemark Abbey Partners Blend, which has a moderate pH of 3.68 and an alcohol content of 14.1%. It likely has the potential to age well.
Unlike some writers, I don’t dismiss young wines for their tartness. If the flavors are balanced, a bit of aging or decanting might be all they need. However, I’m certain that high-pH red wines, which are often too soft, do not age well and are less enjoyable — with or without food.
If today's story captured your interest, explore these related articles:
Celebrating the remarkable life and legacy of Miljenko “Mike” Grgich
Father and son winemakers Daniel and Sam Baron mix old with new
Dan Berger has been writing about wine since 1975.







Great to now include fine wine writer Dan Berger among NVF writers! I recall sharing San Diego papers…he with the San Diego Union and I with San Diego Evening Tribune…We occasionally met at the elevators…And Peter Kilkus’ cool sugar cube idea is fascinating.
Nice article. As a scientist myself I enjoy you tickling my old chemistry button. You could have titled your article "The unseen influence of ph(d) on wine quality." I'm not much of a wine expert, but I do have a trick that "offends" my friends, Jim & Susan, who own Rustridge Winery. It's my soon-to-be-patented "sugar cube wine adjuster". I'm not a fan of dry wines so I keep a box of sugar cubes close at hand. If my bottle of wine is not sweet enough, I simply place a sugar cube on the mouth of the bottle. The cube size is just large enough to fit on top without falling in. Next I place a few drops of water on the sugar cube to saturate it. Then I watch as the sugar cube slowly dissolves and crumbles into the bottle - actually a very satisfying and calming process to watch!